Government regulation applied to the pharmaceutical industries that requires the implementation of systems tools and artificial vision equipment, to guarantee the authenticity of the medicines produced and commercialized, from the laboratory until their acquisition by the customer.

Serialization means assigning a unique random serial number to a sales unit so that it is traceable throughout the supply chain according to the GS1® standard.
Control in real time the drug path and location
Follow up the establishments that commercialize the product.
Reduce falsification or adulteration and illegal drug trading
Reduce financial fraud.
Facilitates product recall.
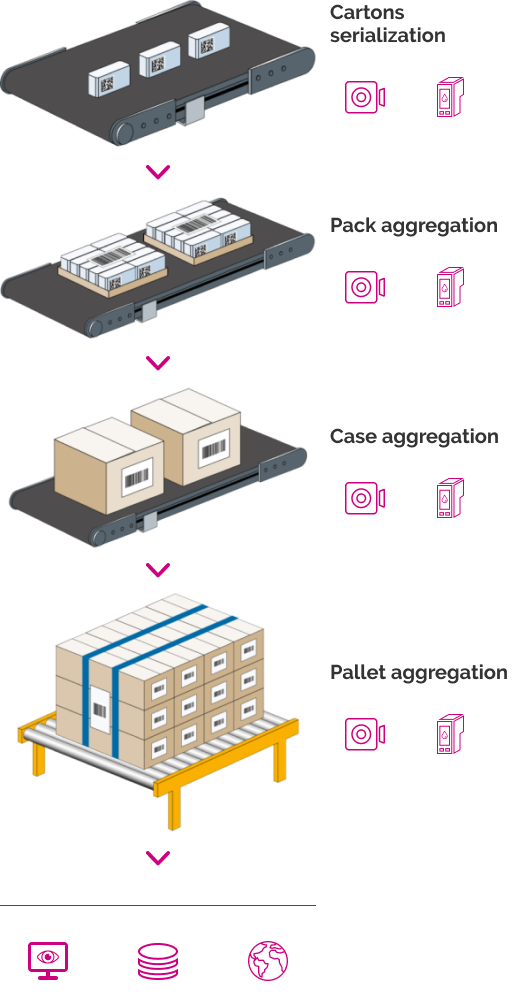
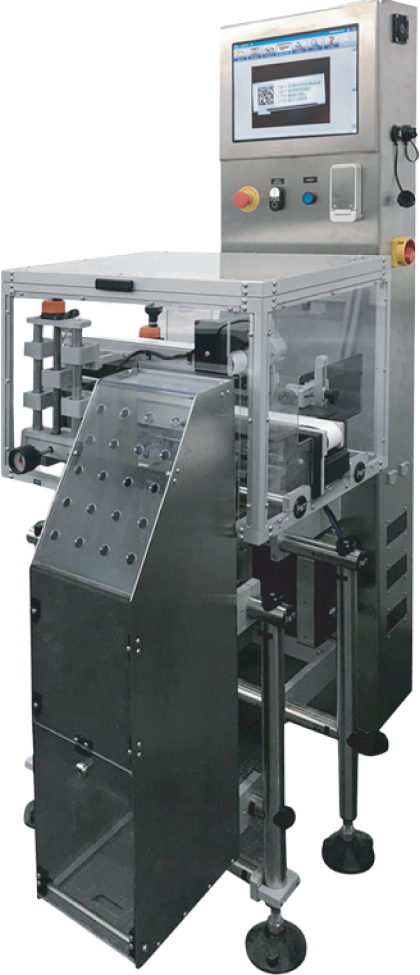
Compact carton serialization module for traceability, integrating printer, inspection camera and reject in a self-contained transport unit.
Small size, suitable for line integration or as a stand-alone manual feeding module.
The equipment interacts with the track & trace system, receiving the batch serial numbers or generating them by itself.
Controls different types of common encoders on the market according to ISO 15415 to print the data on the carton: Datamatrix, GTIN, Serial No., Lot, Expiration, Fabrication, etc.
Updates the information validated in the track & trace management system database: codes, printed, verified, discarded, seized, etc.
Quick and easy format adjustment.
Serialization equipment specially designed for the pharmaceutical industry, integrated with Stand Alone tapes, labelers, Tamper Evident applicators and dynamic scales according to project.



Module for end-of-line Track & Trace control, designed to associate the Datamatrix codes of the cartons with the grouping label (pack, case and pallet).
It performs simultaneous reading of the cases inside a pack or box through a single image or through sequential reading on the machine in special cases.
The equipment interacts with the traceability system by receiving the serial numbers printed on the lot cases or it can build a base by itself based on the reading of the inspected datamatrix.
Generates serialized pack, case and pallet grouping labels and updates the database with the corresponding serial numbers.
Alerts on the presence of products that do not meet the printing quality requirements and/or have erroneous codes.
Compact and modular equipment capable of being integrated into existing lines or Stand Alone modules.
Quick and easy format adjustment.
Modularity
Customizable
Low impact on validation
Integration with ERPS
Integration with approved L4
Modular and scalable solution to minimize the initial investment and guarantee the continuity of the business in the future.
Lists and demonstrable integrations in association with all nationally and internationally recognized Level 4 providers.
Automation of production orders and master data from integration with ERP / MES.
Integración en asociación con OEM reconocidos internacional y nacionalmente.
Efficient integration into existing machines, minimizing implementation time.
SLA tailor-made customer. Warranty on local supplies for 10 years.
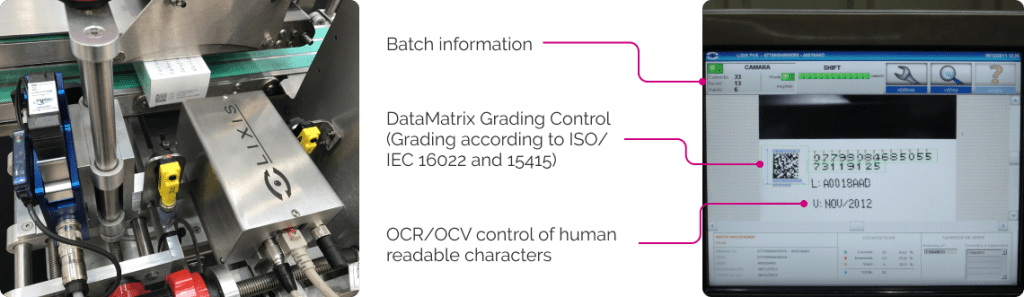
Stand Alone equipment capable of integrating printer and control system for serialization or OCR/OCV control.
The module has a lower and upper band to guarantee the stability of the case, achieving precision in the printing of alphanumeric characters and 2D codes, and the corresponding inspection and discarding of these (OCR/ Grading ISO 15415).
This system is designed to meet the requirements and regulations of the pharmaceutical industry and can be integrated into the existing production line by adapting to upstream and downstream machines, as well as off-line with manual feeding.
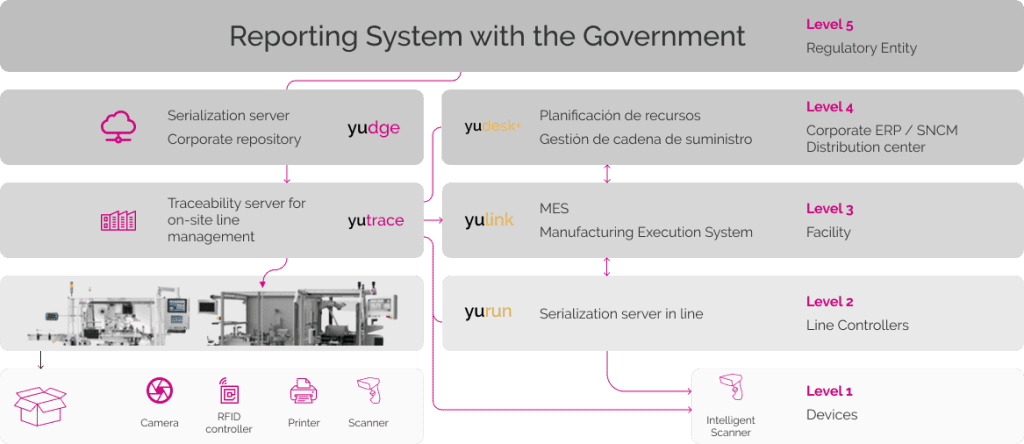
Track & Trace Solution is natively integrated with Yudoo, the 4.0 pharmaceutical software suite for the full management of automation processes, digital quality, data analysis and for Track&Trace. A digital hub that is everywhere accessible through a secure-access web interface or dedicated devices.
A scalable and modular suite, including tools for automation (centralized management of production, workflows and timesheet), for the digital quality (creation of paperless systems, support systems for line clearance operations), for data analysis (analysis of production data, business intelligence dashboard, condition monitoring and predictive maintenance) and for Track and Trace (complete solutions for Level 3 and 4 of serialization, monitoring of serialization operations).
Yudoo offers multiple advantages such as the possibility to centralize production formats and data, avoiding duplicates and reducing work time. The suite can connect to existing company systems to retrieve updated data exactly from where they are.

Lima 1368
Martinez, Buenos Aires
T. +54 11 4836-2800
SÃO PAULO
Rua Mergenthaler 94
Villa Leopoldina, São Paulo
T. +55 11 3798-7800
GOIÁS
Av. María Miguel Abrão, 61 QD.61,
LT.01 Setor Sul Jamil Miguel, Anápolis, Goiás
CEP 75124-720
T. +55 62 3313-5620
Av. Rio Mixcoac 43
Del. Benito Juarez, CDMX
T. +52 55 8796 8004
SEA Vision S.r.l.
via Claudio Treves, 9 E/F
27100 Pavia (PV) – Italy
T. +39 0382 529576
Contact us
NEWSLETTER
Suscribe to our newsletter to receive our news.