Home | Solutions | Pharmaceutical packaging control | Active ingredients control
Near Infrared Hyperspectral System capable of performing geometric analysis and inspection of the chemical composition of the product on the production line. Allows identification of the active ingredients and doses of each product.



Identify drugs with different active ingredients (qualitative analysis).
Identify drugs with different doses or measure the distribution uniformity of their active ingredients (quantitative analysis).
In-line API inspection
Non-destructive analysis
100% of products inspected
Accurate results in real time
Accurate results in real time
Image acquisition rate up to 15,000 frames/sec.
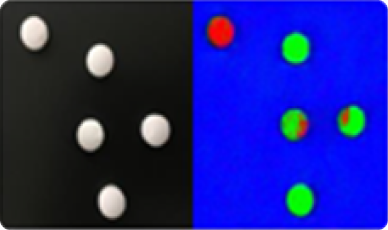
Detection of broken, cracked or chipped tablets and partial or total absence of coating on the product.
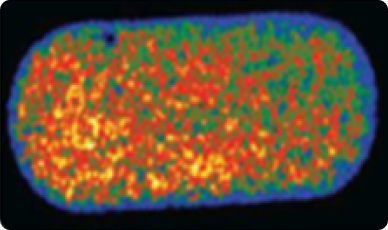
Uniform content. Hyperspectral imaging can be used to analyze the spatial distribution of an active ingredient within the capsule (powder homogeneity).
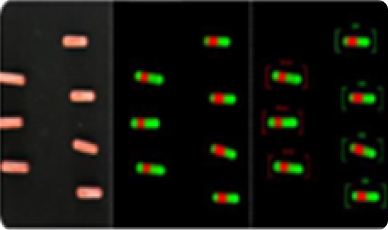
The red stripe illustrates the overlapping area. The capsules on the left have less overlapping area compared to the capsules on the right.
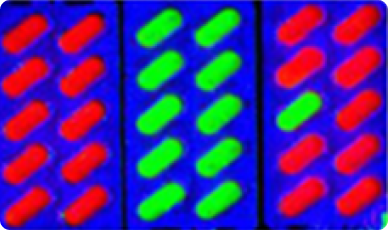
Measurement of the distribution of the active ingredient inside the tablet.
Identification of an empty blister or a blister filled with different tablets (cross-contamination).
Thanks to Near Infrared Hyperspectral Infrared technology it is possible to perform common pharmaceutical quality controls on drugs. The Hyperspectral Infrared system uses chemical compounds present within a particular sample to distinguish and identify materials.
Traditionally QA has relied on laboratory analytical techniques which can now be handled one at a time to obtain 100% safe products.
Spectral range: 900-1700 nm
Spectral band: 224
Spectral resolution: 3.5 nm
Space pixels: 640
Frame rate acquisition: from 670 frames/sec to 15,000 frames/sec
In-depth inspection tablets: about 200 microns for tablets (capsules can pass completely through)
Light unit: halogen light
Accuracy: less than 0.3 mg per 1 g API
Active Ingredient Control is natively integrated with Yudoo, the 4.0 pharmaceutical software suite for the full management of automation processes, digital quality, data analysis and for Track&Trace. A digital hub that can be accessed from anywhere via a secure web interface or dedicated devices.
A scalable and modular suite, including tools for automation (centralized management of production, workflows and timesheet), for the digital quality (creation of paperless systems, support systems for line clearance operations), for data analysis (analysis of production data, business intelligence dashboard, condition monitoring and predictive maintenance) and for Track and Trace (complete solutions for Level 3 and 4 of serialization, monitoring of serialization operations).
Yudoo offers multiple advantages such as the possibility to centralize production formats and data, avoiding duplicates and reducing work time. The suite can connect to existing company systems to retrieve updated data exactly from where they are.

Lima 1368
Martinez, Buenos Aires
T. +54 11 4836-2800
SÃO PAULO
Rua Mergenthaler 94
Villa Leopoldina, São Paulo
T. +55 11 3798-7800
GOIÁS
Av. María Miguel Abrão, 61 QD.61,
LT.01 Setor Sul Jamil Miguel, Anápolis, Goiás
CEP 75124-720
T. +55 62 3313-5620
Av. Rio Mixcoac 43
Del. Benito Juarez, CDMX
T. +52 55 8796 8004
SEA Vision S.r.l.
via Claudio Treves, 9 E/F
27100 Pavia (PV) – Italy
T. +39 0382 529576
Contact us
NEWSLETTER
Suscribe to our newsletter to receive our news.